For years, the shame attached to mental health made people “suffer in silence”. Especially in India, people's perception of mental health is deeply stigmatized. However, active discussions on mental health and growing research on genetic influences on mental illness are slowly shedding the layers of stigma attached to it.

Mental illness is one of the leading causes of non-fatal disease in India. In 2017, 197·3 million people in India had mental disorders with variable severity. The initial efficacy rate for depression is up to 50% only and the remission rate is low. As a result, people often experience long periods of poor management of symptoms and adverse drug events (ADE).
Individual variations in the response and efficacy of antidepressant treatment can be due to environmental factors, physiological factors, psychological factors, comorbidities, and genetic variation. Recently, the therapeutic area of psychiatry has witnessed efforts to achieve more precise treatments by considering genetic variants and their response to medications.
How does genetics influence the metabolism and outcomes in psychiatry?
Existing studies indicate that genes encoding drug-metabolizing enzymes (e.g., CYP2C19, CYP2D6) can significantly affect the response and efficacy of medications. For example, CYP2D6 metabolizes most antipsychotic drugs. The variation in genes coding the enzyme will impact the drug metabolism such as poor, intermediate, and rapid metabolizers.
Therefore, the optimization of drug therapy depends on an individual’s drug metabolism status. So, the Pharmacogenetic test plays a significant role in understanding various levels of an individual's drug metabolism and response.
Here is the list of significant psychiatric drugs and genes which we cover in our Medicamap
| Drugs | Genes |
|
Tricyclic Antidepressants (TCAs) - Desipramine, Imipramine, Clomipramine, Trimipramine, Amitriptyline, Nortriptyline, Doxepin, Protriptyline |
CYP2D6 & CYP2C19 |
| Selective Serotonin Reuptake Inhibitors (SSRIs) - Fluvoxamine, Paroxetine, Citalopram, Escitalopram, Sertraline | CYP2D6 & CYP2C19 |
|
Atypical Antipsychotics - Aripiprazole, Clozapine, Iloperidone, Risperidone, Perphenazine, Pimozide, Thioridazine, Vortioxetine, Olanzapine |
CYP2D6 |
| Selective Serotonin-norepinephrine reuptake inhibitors - Atomoxetine | CYP2D6 |
|
Miscellaneous
Selective Norepinephrine- dopamine reuptake inhibitors - Bupropion |
How do pharmacogenetic tests help to tailor the prescription in psychiatry?
Check this case study published on Pubmed, which explains the use of Pharmacogenetic testing in Psychiatry cases.
A 76-year-old woman presented with Major Depressive Disorder(MDD) with inadequate response to antidepressants, post-traumatic stress disorder, obsessive-compulsive disorder, generalized anxiety disorder, pseudobulbar affect, insomnia, restless leg syndrome, constipation, back pain, gastroesophageal reflux disease, hyperlipidemia, and glaucoma.
The table below shows the medications the primary care provider(PCP) prescribed to manage her various medical conditions.
MDD - Major Depressive Disorder; PTSD – Post-traumatic stress disorder; OCD – obsessive-compulsive disorder; Generalized Anxiety Disorder.
Drug therapy evaluation and optimization
The clinical pharmacist reviewed the patient medication chart and evaluated factors influencing drug response. Although the patient adheres to medications, drug response is limited despite using various antidepressants.
The medications prescribed have vital pharmacogenetic significance. So, the clinical pharmacist recommended a Pharmacogenetic (PGx) test to optimize MDD management. The clinician accepted the recommendation. Her PGx test revealed that the patient has CYP2C19 and CYP2D6 intermediate metabolizers.
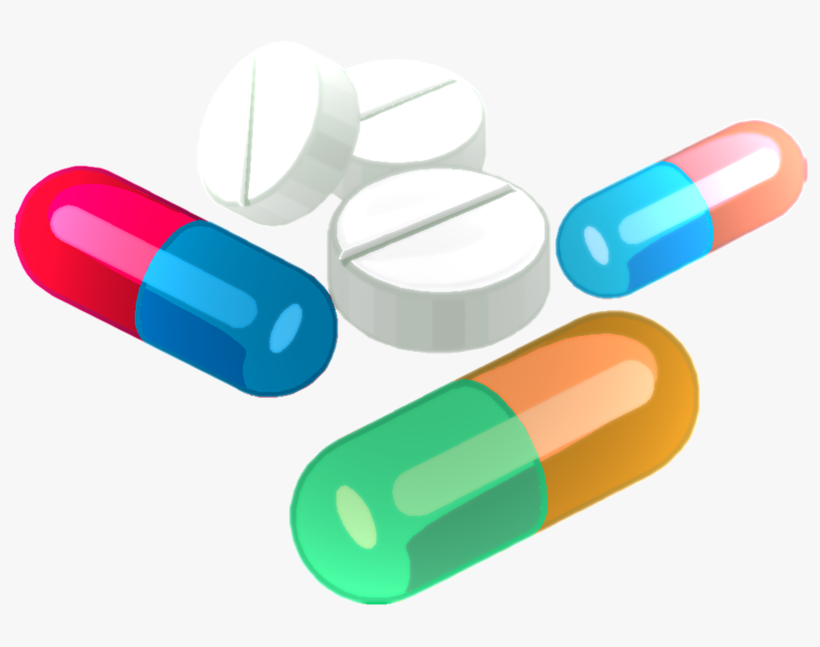
According to Clinical Pharmacogenetics Implementation Consortium(CPIC) guidelines, the drug dosage has been changed, and alternate drugs have been prescribed to the patient. Attempts were made to reduce the dose of quetiapine, and no further dose increase of risperidone was needed. The clinician observed the patient daily while increasing the dose of duloxetine from 30mg to 60mg to optimize antidepressant therapy. The clinician reported that frequent monitoring reduced patient anxiety and improved depression control.
There are more such examples where patients benefited from the Pharmacogenomics tests, which help reduce toxic effects, decrease pill burden, reduce the cost of the therapy, and increase the efficacy.
Consider taking the Medicamap and discover the WHY
Medicamap is an advanced, next-generation comprehensive pharmacogenomic test to assess bio-markers to analyze an individual’s drug-response profile based on their genetic makeup. Medicamap reports the highest clinically relevant genotype–drug associations for over a hundred drug compounds.
The test has been developed for most common drugs used globally (including those prescribed to the Indian population) and covers all FDA-recommended pharmacogenomic specifications.
Medicamap also provides an insight into Phenoconversion. Phenoconversion is a phenomenon that converts genotypic extensive metabolizers (gEMs) into phenotypic poor metabolizers (pPMs) of drugs, thereby modifying their clinical response to that of genotypic PMs. Phenoconversion, usually resulting from non-genetic extrinsic factors, has a significant impact on drug efficacy and Drug-Drug Interactions. Other clinical factors needed to be considered to provide recommendations to optimize psychiatric therapy. This personalized report could guide clinicians to treat mental illnesses.
“The efficacy and tolerability of antipsychotic medications are very low, and the PGx testing would help predict the right medication”
References:
1. The burden of mental disorders across the states of India: the Global Burden of Disease Study 1990–2017 Sagar, Rajesh et al.The Lancet Psychiatry, Volume 7, Issue 2, 148 - 161
2. van Schaik RHN, Müller DJ, Serretti A, Ingelman-Sundberg M. Pharmacogenetics in Psychiatry: An Update on Clinical Usability. Front Pharmacol. 2020 Sep 11;11:575540. doi: 10.3389/fphar.2020.575540. PMID: 33041820; PMCID: PMC7518035.
3. Jha MK, Trivedi MH. Personalized Antidepressant Selection and Pathway to Novel Treatments: Clinical Utility of Targeting Inflammation. Int J Mol Sci. 2018 Jan 12;19(1):233. doi: 10.3390/ijms19010233. PMID: 29329256; PMCID: PMC5796181.
4. van Westrhenen R, Aitchison KJ, Ingelman-Sundberg M, Jukić MM. Pharmacogenomics of Antidepressant and Antipsychotic Treatment: How Far Have We Got and Where Are We Going? Front Psychiatry. 2020 Mar 12;11:94. doi: 10.3389/fpsyt.2020.00094. PMID: 32226396; PMCID: PMC7080976.
5. Krebs K, Milani L. Translating pharmacogenomics into clinical decisions: do not let the perfect be the enemy of the good. Hum Genomics. 2019 Aug 27;13(1):39. doi: 10.1186/s40246-019-0229-z. PMID: 31455423; PMCID: PMC6712791.
6. Del Toro-Pagán NM, Matos A, Bardolia C, Michaud V, Turgeon J, Amin NS. Pharmacist assessment of drug-gene interactions and drug-induced phenoconversion in major depressive disorder: a case report. BMC Psychiatry. 2022;22(1):46. Published 2022 Jan 20. doi:10.1186/s12888-021-03659-4
7. M. Whirl-Carrillo, E.M. McDonagh, J.M. Hebert, L. Gong, K. Sangkuhl, C.F. Thorn, R.B. Altman and T.E. Klein. "Pharmacogenomics knowledge for personalized medicine" Clinical Pharmacology & Therapeutics (2012) Oct;92(4):414-7.