
This case study aims to evaluate the clinical outcome of a SOX3 duplication detected by chromosomal microarray analysis, emphasizing the significance of long-term follow-up and parental segregation in understanding the implications of the duplication.
Family History
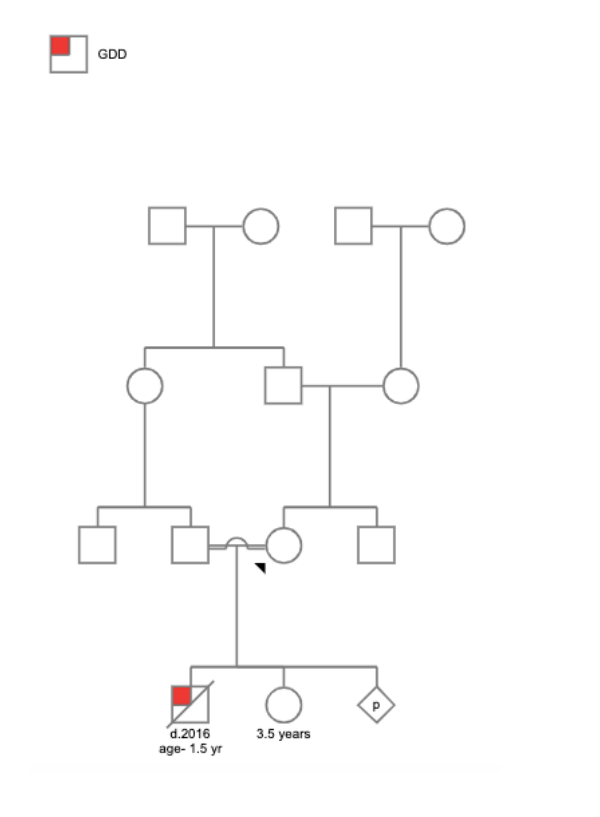
The family presented with a significant family history, including their first child, a son, who exhibited global developmental delay (GDD) and dysmorphism before passing away at 1.5 years of age. Additionally, the family includes a healthy daughter, their second child, now 3.5 years old, who was found to have a duplication on Xq27 through chromosomal microarray analysis. It is important to note that the parents are first cousins, indicating a consanguineous relationship. Furthermore, the family is currently in their second trimester of their third pregnancy.
Pedigree Chart

Indications for testing
The indications for testing in this case were twofold: firstly, to determine the recurrence risk of the duplication detected in the healthy, living daughter, and secondly, to make informed decisions regarding testing their current pregnancy. The family sought genetic testing due to their first child's developmental delay and dysmorphism, as well as the desire to assess the genetic status of the current pregnancy.
Chromosomal Microarray testing and findings
Chromosomal microarray analysis performed during the second pregnancy revealed a heterozygous duplication spanning approximately 3.5MB on the X chromosome at Xq27.1q27.3 in the fetus. This duplication was classified as likely pathogenic. However, at the time of the second pregnancy, parental segregation analysis was not conducted to determine whether the duplication was inherited from a parent. It was only recently, during the mother's (current) third pregnancy, after a span of almost 3.5 years, that parental testing was performed. This long-term follow-up revealed that the mother indeed harbors the same duplication on her X chromosome. Remarkably, both the daughter and the mother, despite carrying the duplication, remain healthy and do not exhibit any clinical symptoms associated with the SOX3 duplication.
The Xq27 duplication
The Xq27 duplication identified in this case is a rare chromosomal aberration. Previous reports have described similar duplications involving the SOX3 gene at Xq27, resulting in a wide range of clinical manifestations. In affected males, hypopituitarism and growth hormone deficiency are commonly observed. However, affected females may exhibit a normal phenotype due to non-random X-inactivation of the duplicated allele. Large duplications encompassing SOX3 have been reported in association with intellectual disability, growth retardation, ocular abnormalities, hypotonia, seizures, and developmental delay.
Recommendations
Based on the genetic findings and the potential for variable penetrance and expressivity of the identified duplication, it is recommended that the family undergo further prenatal chromosomal microarray (CMA) testing for their current pregnancy. Additionally, it is advisable for the family to consider prenatal CMA testing for subsequent pregnancies as well. These recommendations will enable a comprehensive assessment of the fetus's genetic status, providing valuable information for making informed decisions about medical interventions or specialized care.
Conclusion
This case highlights the significance of long-term follow-up and parental segregation in assessing the clinical outcome of a SOX3 duplication detected through chromosomal microarray analysis. The identification of the SOX3 duplication in both the healthy daughter and the mother emphasizes the importance of understanding variable penetrance and expressivity in such cases. It is important to note that while genetic testing can accurately identify the presence or absence of a variant during pregnancy, it cannot however predict the penetrance, severity or phenotypic presentation associated with the variant. Long-term monitoring of the daughter's health and development, along with appropriate genetic counseling, will contribute to a better understanding of the manifestation and progression of this genetic alteration. By offering prenatal CMA testing and ensuring long-term follow-up, healthcare providers can support families in making informed decisions and providing optimal care for affected individuals.

